Dialogue between estrogen receptor and E2F signaling pathways: The
$ 26.00 · 4.9 (686) · In stock

Estrogen receptors and E2F transcription factors are the key players of two nuclear signaling pathways which exert a major role in oncogenesis, particularly in the mammary gland. Different levels of dialogue between these two pathways have been deciphered and deregulation of the E2F pathway has been shown to impact the response of breast cancer cells to endocrine therapies. The present review focuses on the transcriptional coregulator RIP140/NRIP1 which is involved in several regulatory feed-back loops and inhibitory cross-talks between different nuclear signaling pathways. RIP140 regulates the transactivation potential of estrogen receptors and E2Fs and is also a direct transcriptional target of these transcription factors. Published data highlight the complex regulation of RIP140 expression at the transcriptional level and its potential role in transcription cross-talks. Indeed, a subtle regulation of RIP140 expression levels has important consequences on other transcription networks targeted by this coregulator. Another level of regulation implies titration mechanisms by which activation of a pathway leads to sequestration of the RIP140 protein and thus impinges other gene regulatory circuitries. Altogether, RIP140 occupies a place of choice in the dialogue between nuclear receptors and E2Fs, which could be highly relevant in various human pathologies such as cancer or metabolic diseases.

Estrogen receptor - wikidoc

Dialogue between estrogen receptor and E2F signaling pathways: The transcriptional coregulator RIP140 at the crossroads

Mechanisms and significance of nuclear receptor auto- and cross-regulation.

Patrick AUGEREAU, Institut de Recherche en Cancerologie de Montpellier, Montpellier, U896

Cells, Free Full-Text

Regulation of estrogen receptor signaling in breast carcinogenesis
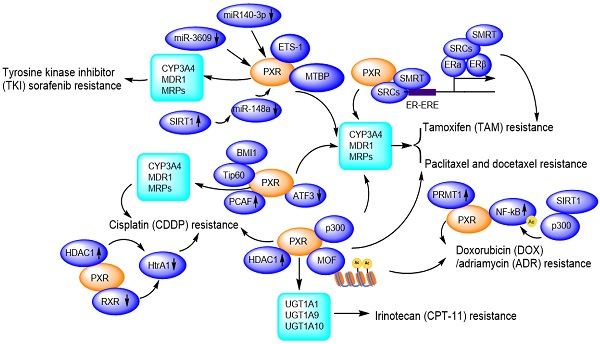
Insights into the critical role of the PXR in preventing

Estrogen receptor signaling mechanisms. - Abstract - Europe PMC

Schematic representation of GIOT-4 function as a gonadotropin-induced