What is the compressibility factor (Z) for 0.02 mole of a van der
$ 20.50 · 4.6 (491) · In stock

Chemical Thermodynamics

Investigation of the Properties of Hydrocarbon Natural Gases Under Confinement in Tight Reservoirs Due to Critical Properties Shift

Compressibility factor (gases) - Knowino
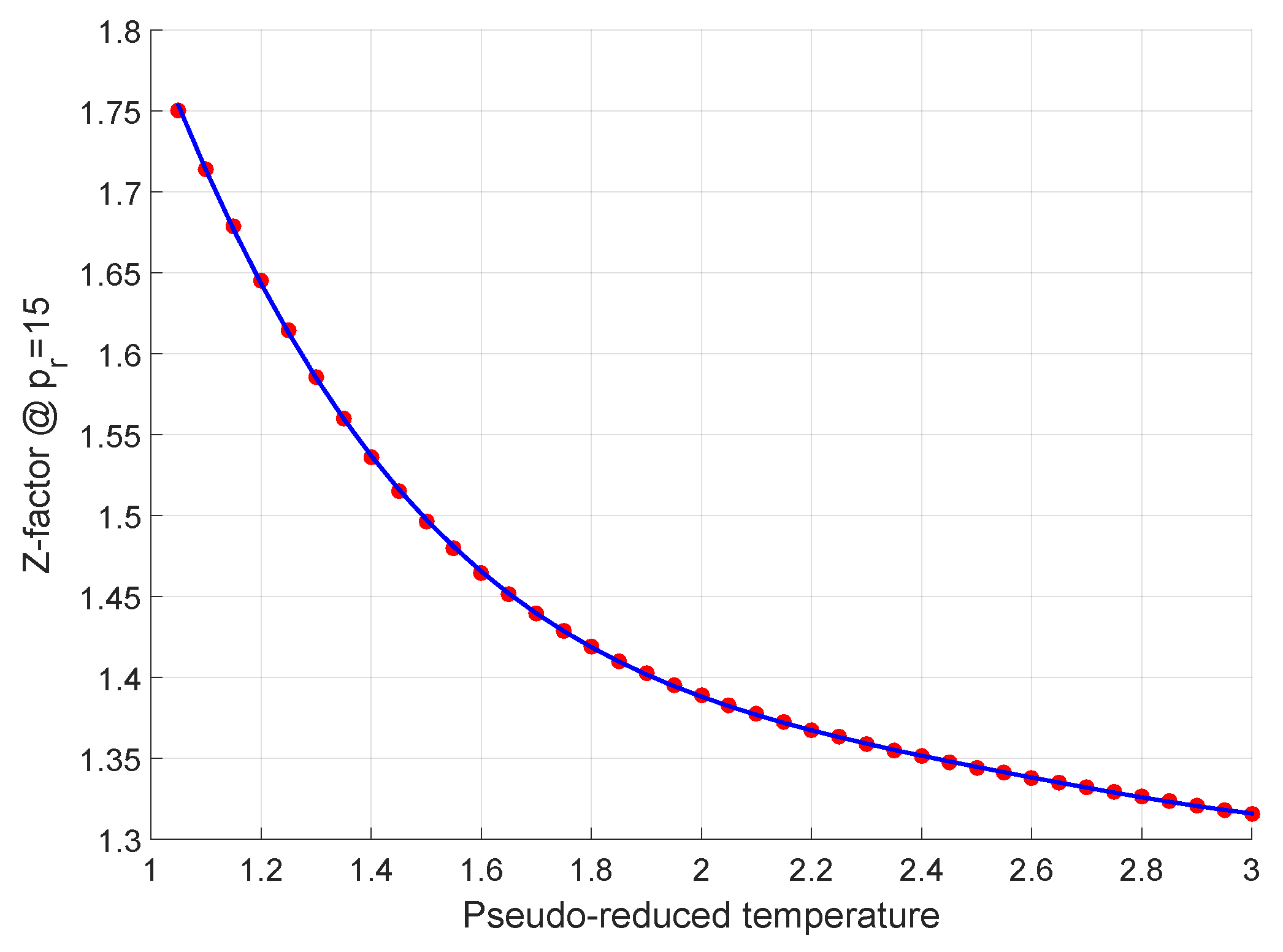
Energies, Free Full-Text

Consider the equation Z=P V/R T. Which of the following statements is correct? (a) When Z1, real

SOLVED: Find the compressibility factor Z for a real gas with the van der Waals equation of state when: a) There are no attractive forces within gas molecules? b) There are no
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

The second virial coefficient obtained from different models for Nitrogen.
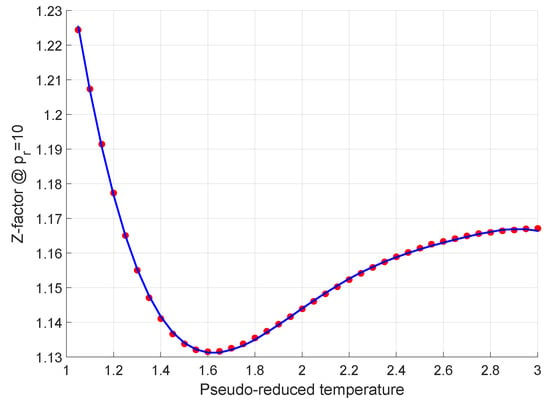
Energies, Free Full-Text

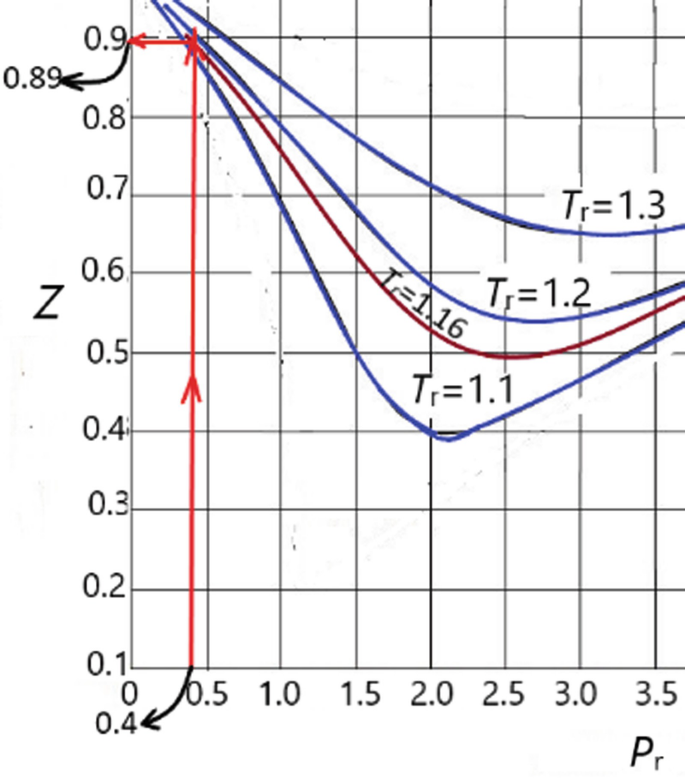
SOLVED: If PR=3, TR=2.0, the compressibility factor (Z) is equal to* 2 Points) a. Z=0.85 b. Z=0.55 c. Z=1.5 d. Z=0.95

Amount of oxygen required for complete combustion of 27 gAl is

Chemical Process Engineering - Harry Silla - Ventech!